All plant cells are surrounded by cell walls, which provide protection from the environment and support during growth and development. Cell walls are important for society because they provide raw materials for energy production (lignocellulosic feedstocks), building materials (wood) and affect food crop yield (resistance against biotic and abiotic stress). Traditionally cell walls have been described as sturdy, solid structures that do not change their composition and structure. Research during recent years has shown that cell walls are actually highly dynamic / plastic structures, which adapt composition and structure in order to meet different functional requirements during development and stress exposure. The plasticity is mediated by the plant cell wall integrity maintenance mechanism, which monitors the functional integrity of cell walls during growth as well as interaction with environment and initiates compensatory responses to maintain integrity. Such a mechanism has been also described in the baker´s yeast, Saccharomyces cerevisiae suggesting that it is a highly conserved mechanism.
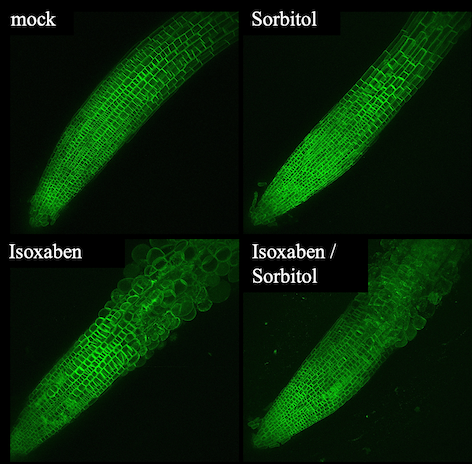
The research group is interested in the mode of action of the plant cell wall integrity (CWI) maintenance mechanism. The group uses Arabidopsis thaliana seedlings as a model system since they have practical advantages like small size and short generation times in addition to a fully sequenced genome, a large number of molecular tools being available and most importantly the knowledge generated is relevant for other plant species. In order to activate the CWI maintenance mechanism we use a chemical (isoxaben), which inhibits production of cellulose, the major load bearing component of plant cell walls and probably most abundant biopolymer on the planet. If cellulose production is inhibited, cells change their shape because of the high levels of turgor pressure (similar to a car tire) pushing against a weakened cell wall. Turgor pressure levels can be reduced by adding Osmoticum (sorbitol), thus preventing shape changes. This is exemplified in the figure below where Arabidopsis seedling roots are treated with isoxaben, osmoticum or combinations thereof. The roots express a tagged protein staining the plasma membrane thus allowing visualizing changes in cell shape (green). We have used this model system to initiate a functional analysis of the molecular mechanisms responsible. Confocal images produced by Nora Gigli-Bisceglia. Currently we are investigating the mode of action of the mechanisms by identifying relevant molecular components using a combination of transcriptomics/phospho-proteomics followed by functional characterization of the candidates. This work is funded by the research council of Norway through the WALLINTEGRITY project. A recent review article from the research group published in Nature Plants provides a global perspective of our work on plant cell wall integrity maintenance.
We have recently shown that the receptor like kinase THESEUS1 (THE1) is required for regulating plant cell wall stiffness in response to cell wall damage caused by Isoxaben. More importantly we also have found that THE1 is modulating production of Abscisic acid (ABA), a signaling molecule found in plants, which regulates adaptation to drought stress (Bacete et al., 2022, see figure below for graphic summary). We are currently following up on these results through the HYDROSENSING ERC SYNERGY project together with colleagues in Germany, the UK and Israel.

In parallel the group is currently hosting two MSCA fellows. One (Dhika Amanda) is using molecular rotors to investigate viscosity changes in Arabdiopsis seedling roots in the context of the cell wall damage perception and osmotic stress (WallABAutstiffness).
The second MSCA fellow (Tereza Ticha) is investigating the transcriptional regulation of primary cell wall metabolism by combining omics based candidate gene selection and functional characterization of the candidates (WallSAtisfaction).
Another area of interest is the mechanism coordinating plant cell wall metabolism with cell cycle activity. Below are images of seedling root tips where cell walls are stained with a red dye while the activity of a cell cycle gene is indicated by green staining. Inhibition by isoxaben causes also shutdown of gene activity, allowing the research group to study the mechanism responsible. We could show that the effects observed require controlled changes in the concentration of cytokinins and require NO-based signaling processes (Gigli-Bisceglia et al., 2018). This area is currently being pursued through a research council young talent project awarded to Laura Bacete-Cano with Nancy Soni pursuing this project in the group.
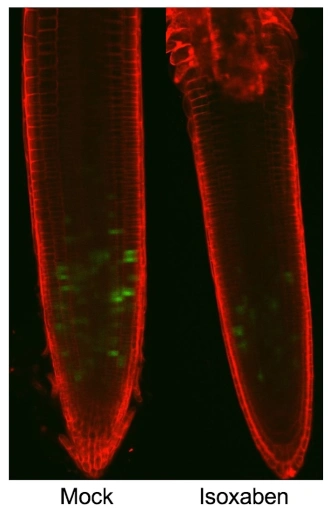
The image shows expression of a GFP-tagged cell cycle marker in mock or isoxaben-treated Arabidopsis root tips.
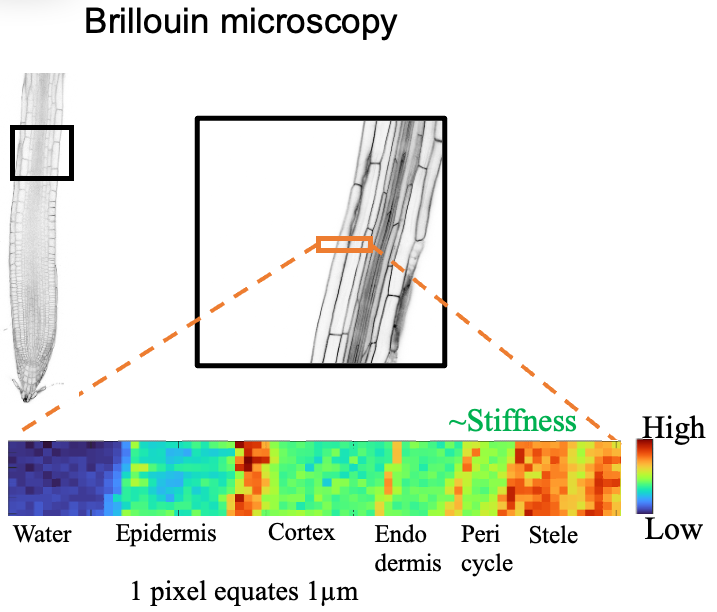
In parallel to the science-driven project going on in the group we are also developing new analytical technologies. This is exemplified by a Brillouin microscope we have built together with colleagues in the physics department. It consists of a Leica SP8 confocal component and a custom Brillouin module. This set up enables us to investigate the mechanical characteristics of tissues and sub-cellular structures in a label-free manner in vivo while simultaneously visualizing fluorescently-labeled proteins. The image below shows part of an Arabidopsis seedling root, where the cell walls exhibit higher stiffness levels (orange-red) while the cytoplasm being less stiff exhibits greenish-blue colors (Image courtesy of Luis Alonso-Baez).
In parallel to our work in Arabidopsis we are also pursuing research projects in Strawberry and in the parasitic plant Cuscuta campestris. In strawberry Steven Zwartkruis is investigating how cell wall integrity maintenance contributes to biotic and abiotic stress adaptation.

In Cuscuta and Arabidopsis/Tomato Wiebke Häger will determine the mechanisms enabling certain cell wall modifying enzymes to contribute to development while also playing important roles in parasite-host interactions.
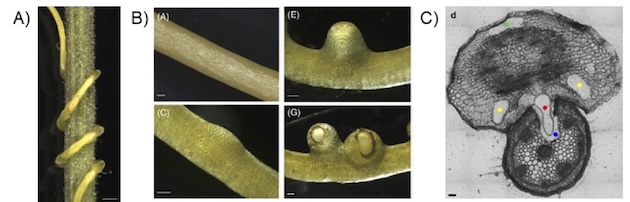
Cuscuta campestris host plant infection and stages of haustorium formation. A) C. campestris infecting tomato, B) C. campestris haustorium formation induced in a host-free system, C) cross-section of C. campestris infecting Pelargonium zonale. A and B adapted from Bawin et al. (2022)13, C adapted from Olsen and Krause (2019)
To summarize the research group is pursuing multiple research projects in the areas of basic and translational science while developing novel tools and technologies to enable leading edge research in plant biology.
Selected publications from the group:
Bacete L., Schulz J., Engelsdorf T., Bartosova Z., Vaahtera L., Yan G., Gerhold JM., Ticha T., Ovstebo C., Gigli-Bisceglia N., Johannessen Starheim S., Margueritat J., Kollist H., Dehoux T., McAdam Sam, Hamann T. «THESEUS1 modulates cell wall stiffness and abscisic acid production in Arabidopsis thaliana» PNAS, 119, 1, 2022.
Parry G, Provart NJ, Brady SM, Uzilday B; Multinational Arabidopsis Steering Committee. “Current status of the multinational Arabidopsis community.” Plant Direct, 4 (7): e00248, 2020.
Bacete L, Hamann T. “Turn down the volume: how plants respond to cell swelling” Current Biology, 30 (14), R804-R806, 2020.
Bacete L, Hamann T. “The role of mechano-perception in plant cell wall integrity maintenance” Plants, 9(5):E574, 2020.
Kimura S., Kerri Hunter K., Vaahtera L., Tran C., Vaattovaara A., Rokka A., Stolze S.C., Harzen A., Meißner L., Wilkens M., Hamann T., Toyota M., Nakagami H., Wrzaczek M. “CRK2 and C-terminal phosphorylation of NADPH oxidase RBOHD regulate ROS production in Arabidopsis“ Plant Cell, 32 (4), 1063-1080, 2020.
Gigli-Bisceglia N. and Hamann T. “Plant cell wall integrity maintenance in model plantsand crop species-relevant cell wall components and underlying guiding principles” Cellular and Molecular Life Sciences, 77 (11), 2049-2077, 2020.
Vaahtera l., Schulz J., Hamann T. “Cell wall integrity maintenance during developmentand interaction with the environment” Nature Plants, 5 (9), 924-932
Engelsdorf T., Kjaer L., Gigli-Bisceglia N., Vaahtera L., Miedes E., Bauer S., Wormit A., James L., Chairam I., Molina A. and Hamann T. Functional characterization of genes mediating responses to plant cell wall integrity impairment and cell wall metabolism. 2019 BMC Plant Biology
Gigli-Bisceglia N., Engelsdorf T., Strnad M., Vaahtera L., Khan GA., Yamoune A., Alipanah L., Novák O., Persson S., Hejatko J. and Hamann T. Cell wall integrity modulates Arabidopsis thalianacell cycle gene expression in a cytokinin- and nitrate reductase-dependent manner. 2018 Development
Engelsdorf T., Gigli-Bisceglia N., Veerabagu M., McKenna JF., Augstein F., van der Does D., Zipfel C. and Hamann T. The plant cell wall integrity maintenance and immune signaling systems cooperate to control stress responses in Arabidopsis thaliana. 2018
Gigli-Bisceglia, N. and Hamann T. Outside-in control – Does plant cell wall integrity regulate cell cycle progression? 2018 Physiologia Plantarum
Paniagua C., Bilkova A., Jackson P., Dobrowolski S., Riber W., Didi V., Houser J., Gigli Bisceglia N., Wimmerova M, Budínská E., Hamann T. and Jan Hejatko. Dirigent proteins in plants – modulating cell wall metabolism during abiotic and biotic stress exposure. 2017 JExBot
Van der Does D., Boutrot F., Engelsdorf T., Rhodes J., McKenna JF., Vernhettes S., Koevoets I., Tintor N., Veerabagu V., Miedes E., Segonzac C., Roux M., Breda AS., Hardtke CS., Molina A., Rep M., Testerink C., Mouille G., Höfte H., Hamann T. and Zipfel C. The Arabidopsis leucine-rich repeat receptor kinase MIK2/LRR-KISS connects cell wall integrity sensing, root growth and response to abiotic and biotic stresses. 2017 PLOS Genetics
Hamann T. The plant cell wall integrity maintenance mechanism – Concepts for organization and mode of action. 2015 Plant and Cell Physiology
Hamann T. The plant cell wall integrity maintenance – A case study of a cell wall plasmamembrane signaling network. 2015 Phytochemistry
Engelsdorf T. & Hamann T. “An update on receptor-like kinase involvement in plant cell wall integrity maintenance. 2014 Annals of Botany
Wormit A., Butt S., Chairam I., McKenna J., Nunes-Nesi A., Fernie A. Barter L., Woscholski & Hamann T. Osmosensitive changes of carbohydrate metabolism in response to cellulose biosynthesis inhibition. 2012 Plant Physiology
Hamann T. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. 2012 Frontiers in Plant Science
Denness, L., McKenna JF., Segonac C., Wormit A., Madhou P., Bennett M., Mansfield J., Zipfel, C. Hamann T. The plant response to cell wall damage is regulated through interaction of ROS and JA mediated processes. 2011 Plant Physiology
Hamann T., Bennett M., Mansfield M. & Chris Somerville Identification of cell wall stress as a hexose-dependent and osmosensitive regulator of plant responses. 2009 Plant J.